

首页 / 产品和服务 / 产品类型 / 抗体产品 / 特色抗体推荐
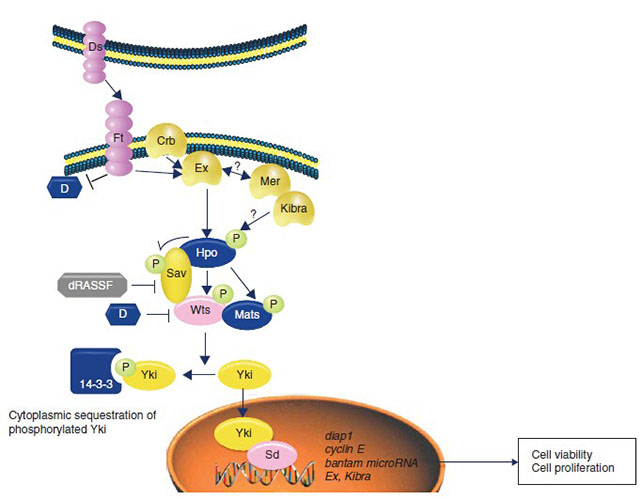
In mammals, the cores of Hippo pathway consist of the mammalian sterile20-like kinases serine/threonine kinases 1/2(MST1, MST2), the large tumor suppressor serine/threonine protein kinases 1/2(LATS1/2), as well as their scaffold proteins Salvador homologue 1(SAV1;also called WW45) and Mps One Binder kinase activator proteins(MOBs). Mechanistically, MST1/2 phosphorylate and activate LATS1/2 complexed with SAV1 and MOB1[11, 12]. Then, activated LATS1/2 phosphorylate the transcriptional regulators, including yes-associated protein(YAP) and transcriptional coactivators with PDZ-binding motif(TAZ)(the major effectors of Hippo pathway). Phosphorylated YAP/TAZ lose their activation by sequestering in the cytoplasm via interaction with 14-3-3 proteins, then are degraded through the ubiquitin-proteasome pathway[13]. On the other hand, as transcriptional regulators, YAP/TAZ bind to multiple transcription factors, such as TEADs, Smads, PAXs, TBX5 and RUNX2[14], and regulate genes involved in stemness, differentiation, proliferation, and apoptosis upon nuclear translocation.
| Main factors | Drosophila melanogaster | Mammals |
|---|---|---|
| The upstream regulators | Mer | Nf2 |
| The core factors | Hippo,Sav,Mats,Wts | Mst1/2,Sav1,Mob1,Lats1/2 |
| The downstream effectors | Yki | YAP/TAZ |
YAP/TAZ are critical during early embryonic development. Although TAZ knockout mice are viable, YAP knockout mice die at E8.5, and blastomeres stop dividing before the morula (16–32 cells) stage when YAP and TAZ are both deleted[15]. Therefore, the role of YAP/TAZ in early development is partially overlapping. The first cell fate specification during embryogenesis occurs during preimplantation stage, in which the trophectoderm (TE) and inner cell mass (ICM) are formed. The TE consists of the outer cells of the blastocyst and forms extraembryonic tissues, while the ICM contain the inner cells of the blastocyst and give rise to the embryo proper and other tissues. The formation of the TE and ICM are mainly due to the position or polarity of cells in the morula, in which the inner and apolar cells form the ICM, while the outer and polar cells give rise to the TE[16]. As early as the 16-cell stage, YAP/TAZ already show differential subcellular localization between inner and outer cells, and this difference lasts to the blastocyst stage. The different distribution of YAP/TAZ in the TE and ICM results in different gene expression signatures, especially the induction of TE-specific genes, such as Cdx2 in outer cells, thus directing cell fate specification[17]. Indeed, mouse embryos with TEAD4 knockout failed to develop TE cells, with all cells differentiating into ICM. On the other hand, depleting LATS1/2, NF2, or AMOT/AMOTL2 turns all cells into TE linage, and these embryos fail to develop ICM-derived tissues[18]. These results suggest that the Hippo pathway plays a key role in early embryonic cell specification.
The effect on organ size is the best-known physiological function of the Hippo pathway. In Drosophila, mutation of Hippo pathway kinases (hpo and wts) or upstream regulators (Ex, Mer, Kibra, Ft, etc.) leads to overgrowth of organs such as eyes, wings, or other appendages, and transgenic expression of Yki results in a similar phenotype. The increased tissue/organ size is mainly due to Yki-induced cell proliferation and survival.
The effect of the Hippo pathway on organ size is highly conserved in mammals, as revealed by many studies performed in mice. For instance, liver-specific transgenic YAP expression in mice can produce a dramatically enlarged liver. Remarkably, the liver returns to its normal size via apoptosis once the overexpression of YAP is turned off [19]. Similarly, liver-specific knockout of Mst1/2, Sav1, or Nf2 also results in liver enlargement[20]. The mouse embryonic heart is enlarged when Sav1, Mst1/2, or Lats1/2 is deleted, and proliferation or apoptosis of cardiomyocytes is sensitive to genetic manipulation of YAP.
High YAP/TAZ activity has been observed in the stem or progenitor cells of multiple tissues, suggesting a role for YAP/TAZ in stem cell maintenance. For example, YAP is highly nuclear in basal progenitor cells and in intestinal stem cells localized at the crypt base[21]. Activation of YAP, either by transgenic expression of YAP or deletion of upstream regulators, usually results in expansion of progenitor cells, impaired cell differentiation, and hyperplasia of target tissues such as intestine, liver, skin, and nervous system[22]. The role of YAP/TAZ on cell proliferation and stem cell expansion suggests a critical function of YAP in normal tissue development and homeostasis. Indeed, tissue-specific deletion of YAP results in abnormalities of the heart, skin, and kidney[23]. However, mammary glands and the intestine remain relatively normal upon YAP deletion [22]. These findings suggest that YAP is required for development and homeostasis of some, but not all, tissues in mice. In human, TEAD1 mutations are found in Sveinsson chorioretinal atrophy, a disease characterized by chorioretinal degeneration, and Aicardi syndrome, a congenital neurodevelopmental disorder[24]. In addition, loss-of-function mutations of YAP have been identified in both isolated and syndromic optic fissure closure defects. Hence, the loss of TEAD-mediated YAP transcriptional activity plays a role in some degeneration-related disorders in humans.
Increasing evidence suggests that the Hippo pathway is dysregulated in many hunman cancers. With the abnormal Hippo pathway, the cells will proliferate excessively or apoptosis insufficiently, and the organ will grow out of control, which will eventually lead to tumorigenesis and tumor progression[25]. Besides the important roles of YAP/TAZ in tissue regeneration and development, elevated YAP/TAZ expression or nuclear enrichment of YAP/TAZ also have been detected in various tumors, such as hepatocellular carcinoma(HCC)[26], breast cancer[27], prostate cancer[28], non-small cell lung cancer[29] and ovarian cancer[30]. However, the majority of cancers with high YAP/TAZ activity have not been linked to genetic mutations of the Hippo pathway, and the overall genetic alteration rate of Hippo pathway components in human cancer is relatively low. One well-characterized example of a Hippo pathway mutation associated with cancer is in NF2, which causes neurofibromatosis 2 lesions, including schwannomas and meningiomas[31]. Moreover, inactivating NF2 mutations are also observed in 40%-50% of malignant mesothelioma[32]. The gene amplification of YAP are detected in hepatocellular carcinomas, medulloblastomas, and esophageal squamous cell carcinomas[33-36]. Besides the mutation, gene fusions involving YAP or TAZ have also been discovered in human cancers. Remarkably, virtually all epithelioid hemangioendotheliomas contain gene fusions of TAZ-CAMTA1, TAZ-FOSB, or YAP-TFE3[37-40]. In addition, YAP gene fusions with MAMLD1 or C11orf95 have been discovered in a subset of ependymal tumors[41, 42]. Sporadical LATS1/2 mutations or gene fusion have been identified in different cancers, which may lead to YAP/TAZ activation. On the other hand, recent studies showed that YAP is constitutively activated in GNAQ- or GNA11-mutated uveal melanomas, and the high YAP activity contributes to tumor growth[43, 44]. The conclusion are as following:
| Gene | Alteration | Cancer type |
|---|---|---|
| NF2 | Mutation or deletion | Mesothelioma |
|
Neurofibromatosis type2 (schwannoma, meningioma) |
||
| LATS1/2 | Gene fusion(LATS1-PSEN1) | Mesothelioma |
| LATS2 deletion | ||
| LATS1/2 mutations | Sporadic in different cancers | |
| YAP | Amplification | Hepatocellular carcinoma |
| Medulloblastoma | ||
| Esophageal squamous cell carcinoma | ||
| Mutation(R331W) | Lung adenocarcinoma | |
|
Gene fusion(YAP-TFE3, YAP-ESR1, YAP-C11orf95, and YAP-MAMLD1) |
Epithelioid hemangioendothelioma | |
| luminal breast cancer | ||
| ependymal tumors | ||
| Deletion | Hematological cancer | |
| TAZ |
Gene fusion(TAZ-CAMTA1 and TAZ-FOSB) |
Epithelioid hemangioendothelioma |
| GNAQ/GNA11 | Activating mutation | Uveal melanoma |
The Hippo pathway is involved in a variety of biological functions regulation, and its dysregulation leads to some diseases, especially human cancers. Hence, prevention and treatment of human malignancies via manipulation of Hippo signaling is a complex prospect.
The YAP/TAZ-TEAD complexes
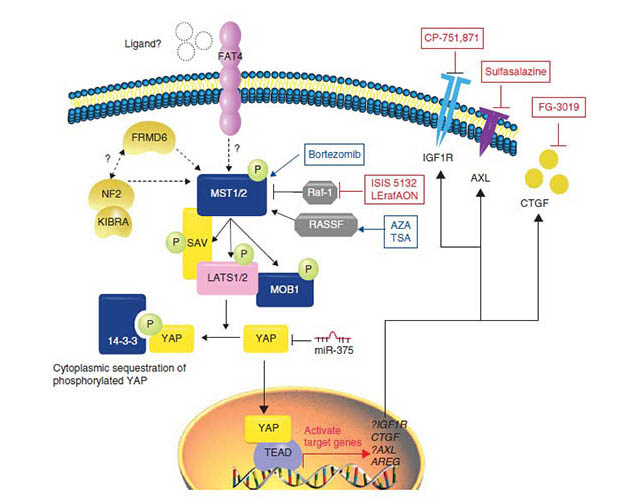
The most thoroughly studied downstream mediators of the Hippo pathway are the YAP/TAZ-TEAD transcription complexes. Small-molecule drugs were identified that interfere with the interaction between YAP/TAZ and TEAD. A screen of 3300 drugs, revealed that members of the Porphyrin family of molecules, including Verteporfin, Hematoporphyrin(HP), and Protoporphyrin IV can prevent the association of YAP and TEAD and subsequently inhibit transcription of their targets[45, 46]. Molecules that compete with YAP for access to TEAD can also modulate YAP-dependent transcription. Recently, it has been shown that Vestgial-Like Family Member 4(VGLL4) directly competes with YAP for binding TEADs through tandem Tondu domains[47, 48], suggesting an alternate possibility for therapeutic YAP inhibition. A peptide mimic of VGLL4 inhibits gastric tumor growth and proliferation in vivo and in vitro[48]. These studies demonstrate that it is the potential therapy through disruption of YAP/TAZ-TEAD interaction.
Kinases
Kinases are common targets for small-molecule therapeutics. However, small molecule inhibitors of the core Hippo pathway kinases MST1, MST2, LATS1 and LATS2 as tumor suppressors would be ineffective. So, focusing on other kinases that modulate this pathway including Homeodomain-interacting protein kinases 2(HIPK2), Proto-oncogene tyrosine-protein kinase Yes(YES1), and Integrin linked kinase(ILK) may have therapeutic potential. Serrano et al. reported that dasatinib, a tyrosine kinase broad-spectrum inhibitor could inhibit the β-catenin-Yap driven cancers via inhibition of YES1[49]. In addition, inhibition of ILK in prostate, breast and colon tumor cells leads to activation of the core kinases MST1 and LATS resulting in inactivation YAP/TAZ-mediated transcription[50].
Mask and WBP2 proteins
Mask is a large protein that contains two blocks of ankyrin repeats as well as a KH RNA binding domain. It is conserved from Drosophila to humans and has two homologues Mask1 and Mask2. It is reported that knockdown of MASK1 may destroy the interaction between YAP and TEAD3 leading to inhibit transcription of YAP target genes CTGF, ANKRD1, FN1, CY61 while non-target genes such as KRAS, NRAS and MYC, YAP and TAZ themselves were not affected. All these data indicate that MASK1 is required for full activity of YAP/TEAD[51]. The overexpression of MASK was observed in acute leukemias[52] and its expression is associated with poor prognosis of breast cancer[51]. On the other hand, the low expression of MASK1 is related to significantly better relapse-free survival in two inde-pendent data sets. These findings suggest that MASK1 levels are an important factor related to YAP in cancer progression and patient outcomes[51]. WW domain-binding protein 2(WBP2) is another interacting protein for YAP and TAZ. The mammalian Wbp2 has three PPXY motifs one of which binds to TAZ. Knockdown of endogenous Wbp2 suppresses TAZ-driven cell transformation and gene expression, whereas over-expression of Wbp2 enhances these processes[53].
Other signaling
(1)EGFR-PI3K signaling
Recently, it is reported that epidermal growth factor receptor(EGFR) inhibits the Hippo pathway via activation of PI3-kinase(PI3K) and phosphoinositide-dependent kinase(PDK1), independent of AKT activity. The PI3K-PDK1 pathway also facilitates translocation of YAP into nucleus. PDK1 physically associates with the core kinase complex of Hippo pathway and dissociates in response to EGF signaling in a PI3K-PDK1-dependent manner, resulting in LATS inactivation and YAP nuclear accumulation[54]. Therefore, inhibitors of EGFR/PI3K signaling may help attenuate YAP activity in tumorigenesis.
(2)GPCR pathway
G protein-coupled receptors(GPCRs), also known as seven-transmembrane domain receptors, constitute a large family of proteins that translate extracellular signals into functional cellular outcomes. Deep sequencing data suggest that 20% of human malignancies have GPCR mutations[55]. Recently, studies have shown that Hippo pathway activity is broadly mediated by GPCR signaling. Serum-borne lysophosphatidic acid(LPA), sphingosine 1-phosphate(S1P) and thrombin act to inhibit LATS1/2 and activate YAP/TAZ transcriptional activity through G12/G13-coupled receptors[56]. Hence, antagonizing LPA, S1P and thrombin-mediated receptor signaling may suppress YAP/TAZ activity in cancer[57]. High specificity monoclonal antibodies have been developed to these molecules[58, 59]. The Sphinaomab(S1p-blocking antibody) has been reported to decrease lung tumor metastasis[60]. The isoflavone-derivative SPHK1 inhibitor, Phenoxodiol, is in clinical trial in patients with platinum/taxane-refractory/resistant ovarian cancers, fallopian tube, or primary peritoneal cancers[61]. In conclusion, the drugs targeting GPCR, LPA, S1P and thrombin represent promising therapeutic approaches for modulating the Hippo pathway.
(3)Mevalonate pathway
The mevalonate pathway is a critical metabolic pathway whose role is synthesis of sterol isoprenoids(i.e. cholesterol) and non-sterol isoprenoids(i.e. dolichol) involving in cellular growth and differentiation[62]. HMG-CoA reductase is the key rate-limiting enzyme of the mevalonate pathway, which regulates YAP phosphorylation and activity and is required for proliferation of breast cancer cells. Interestingly, mevalonate’s control of YAP phosphorylation is independent of core hippo kinase(MST and LATS) activity[63]. Inhibition of HMG-CoA reductase by statins prevents YAP/TAZ nuclear localization and transcriptional responses[64]. Mevalonate and hippo pathways also regulate another tumor-related target, Hyaluronan-mediated motility receptor(HMMR), also known as RHAMM. In breast cancer cells, YAP regulates RHAMM transcription and consequently cell migration and invasion[63]. YAP-mediated control of RHAMM transcription and subsequent metastasis require geranlgeranylation, Rho GTPase activity, and the actin cytoskeleton.
The Hippo pathway has various biological functions including organ size control, tissue regeneration and stem cell self-renewal[1, 2]. The deregulation of Hippo pathway core components, and upstream regulators along with hyperactivation of YAP and crosstalk with other signaling pathways have been linked to tumorigenesis in many ways. There is a great deal of new information on the role of YAP in tumorigenesis. Inhibition of the YAP/TAZ-TEAD complexe is a promising anticancer approach for YAP-dependent tumors. Importantly, inhibition of the GPCR, EGFR-PI3K, and Mevalonate pathways are also attractive anti-tumor therapeutic concepts.
However, one drawback of targeting the pathways is thecytotoxic side effects associated with inhibition of major regulators that can effect critical pathways in normal cells as well. For example, GPCRs have broad physiological functions and interference with their signaling may not be feasible. Similarly, targeting the core Hippo kinases may be problematic. Concentrating on tissue-specific regulation of the Hippo pathway may help reduce side effects and promote therapeutic value. Therapeutic approaches are progressing from pre-clinical to the market place. We anticipate more insights into Hippo pathway regulation will eventually allow us to use these molecules for prevention and treatment of human malignancies.